MILLIKAN OIL DROP EXPERIMENT
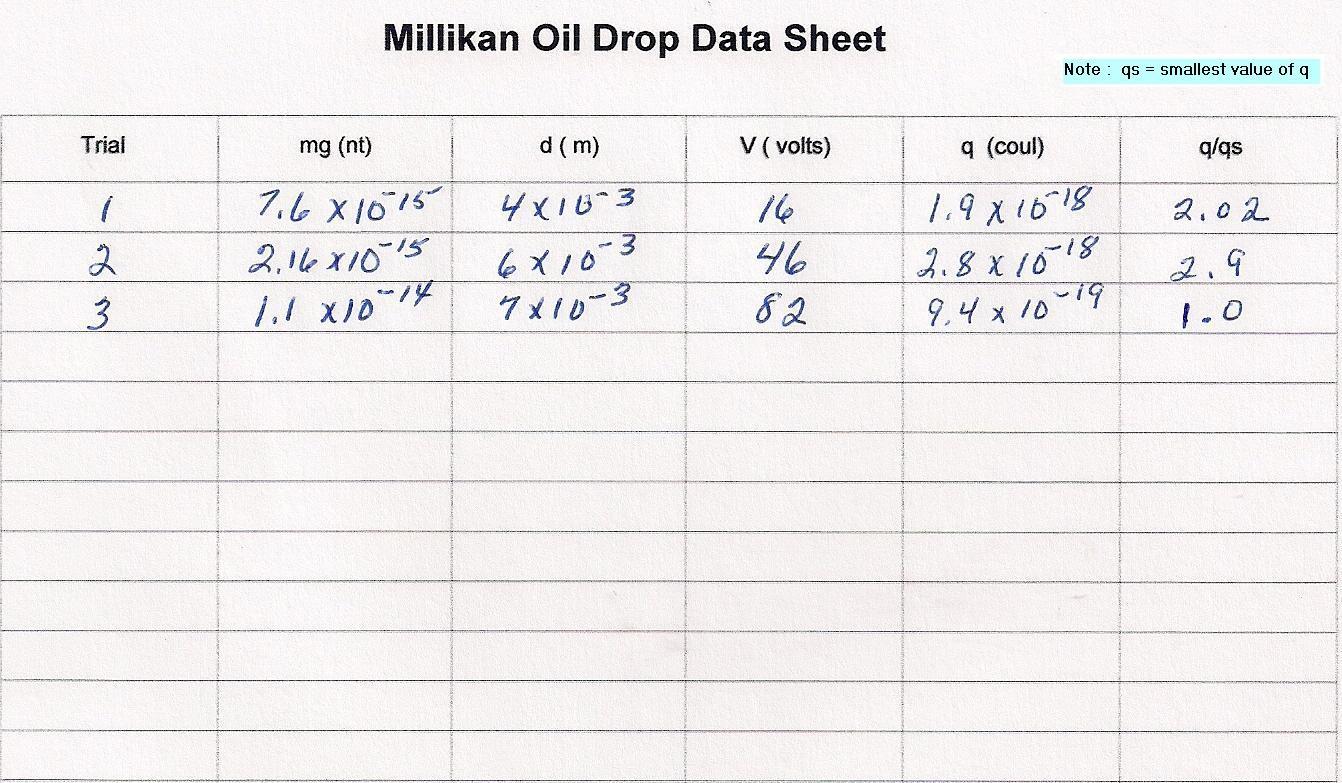

This simulation has been designed to eliminate some of those problems. Millikan had to calculate the weight of the oil drop from the geometry of the drop and the density of the oil. In this simulation you are given the weight of the oil drop in newtons. However, the charge on the oil drop changes from drop to drop. The distance between plates also changes from drop to drop.
We suggest you read the full details of the Millikan experiment as given in Wikipedia.
Millikan was seeking a elementary unit of charge. He did this by suspending an oil drop in an Electric Field so that the force of the Electric Field was equal to the Gravitational Force. He was then able to calculate the charge on the oil drop.